L
lollipop
Guest
+1 They are.Your posts are so good travis.
Follow along with the video below to see how to install our site as a web app on your home screen.
Note: This feature may not be available in some browsers.
Click Here if you want to upgrade your account
If you were able to post but cannot do so now, send an email to admin at raypeatforum dot com and include your username and we will fix that right up for you.
+1 They are.Your posts are so good travis.
How? Did they shine a light through a mouse's optic nerve or something?Photons were found to collapse at retina, not all the way into brain. Hameroff had to work around this.
Diffusion in insufficient to account for vision and nerve conduction. The only long-range structures in the body are proteins; so naturally, these must be the conduits of information.Modern biology is a molecular biology. The rates of most biological reactions are assumed to be limited by the classical concepts of mass action theories applicable to reacting molecules in solution. The main bearers of life are the protein macromolecules, which are often to be found incorporated into membrane structures. This situation would appear to violate the physical basis of classical mass action theories and suggests that the functioning of such structural proteins is controlled at the submolecular level. ―Szent-Györgyi⁽¹⁰⁾
Present biology is a molecular biology. According to it, the main bearers of life are the protein macromolecules with their molecular reactions. One may wonder how such poorly reactive clumsy macromolecules could bring about those subtle biological reactions which characterize life and lend its charm to biology. One may wonder whether these macromolecules are really the main actors of life and whether the main actors are not very much smaller and mobile units, electrons, while the macromolecules themselves are rather the stage than the actors of the drama of life. The problem is whether the electrons of proteins could achieve a greater mobility, lending a subtle reactivity to the protein. ―Szent-Györgyi
But the globular proteins which diffuse freely in the blood are essentially nonconductive. His experiments with casein showed a very low conductivity:
These are extremely-high value for resistivity, similar to rubber, but he was still optimistic. He thought that perhaps the peptide backbone of the protein could be made semiconductive by the addition or removal of electrons, in the manner of ceramic semiconductors. This idea was taken seriously, and mathematical models were developed.⁽¹⁰⁾⁽¹³⁾With a Keithley 600 A electrometer, the resistivities of several casein-methylglyoxal samples were determined to be in the range 44-99 GΩ·m at 295 K. When 200 V were applied across the white casein samples, the steady-state currents attained were less than 0.3 pA, corresponding to resistivity values exceeding 50 TΩ·m. Measured in this way, the resistivity of the casein–methylglyoxal complex was some three orders of magnitude less than that of the white casein under the same atmospheric conditions. ―Szent-Györgyi⁽⁸⁾
It was envisaged that the regular arrangement of peptide linkages in the proteins could result in the existence of electronic energy bands similar to those in elemental semiconductors. Theoretical molecular orbital calculations and experimental studies have supported the validity of this general concept, although it is clear that the energy gap between the so-called valence and conduction bands is too large for pure proteins to act as intrinsic semiconductors. However, this does not preclude the proteins' being able to exhibit a significant extrinsic semiconductivity, through the incorporation of an electron-accepting molecule in their structures, for example. Furthermore, because of the large energy band gap, the action of just one electron acceptor molecule in creating a positively charged "hole" in the protein molecule's valence band could be biologically significant, because there will be no masking effect arising from the intrinsic thermodynamic generation of such charge deficiencies. We wish to report on the electronic properties of one such protein-electron acceptor entity, namely those of the casein–methylglyoxal complex. ―Szent-Györgyi⁽⁹⁾
But he had hopes for the longer structural proteins, although it had been conceded that globular proteins were practically nonconductive.. Such considerations of the reactivity of living systems led one of us to suggest nearly 40 years ago that the functioning of proteins should be understood by considering their submolecular properties. It was envisaged that one manifestation of such submolecular processes would be the ability of proteins to sustain electrical conductivity. However, as shown by the pioneering studies of Eley, proteins isolated in their pure and dry condition are poor conductors. ―Szent-Györgyi⁽¹⁰⁾
This duality in the nature of proteins has, till now, not been fully appreciated. When I proposed, more than 30 years ago (4), that proteins may be semiconductors, the main and apparently decisive argument against my proposition was that none of the great number of proteins isolated in crystals showed any signs of semiconductivity. It was overlooked that crystalline proteins have to belong to the soluble group and so cannot be expected to be semiconductors. It is the structures that are semiconductors, which cannot be crystallized. ―Szent-Györgyi⁽⁶⁾
But even structural proteins were shown essentially nonconductive. He was forced to concede:However, last year Banga and I found that the proteins building up the solid structure of the cell are fibrous, and that these fibrous molecules as shown by their strong thixotropy, are interconnected by inter- molecular forces. Chloroplasts also contain fibrous proteins. This finding allows us to suppose tentatively that a greater number of molecules may join to form such energy continua, along which energy, viz., excited elec- trons, may travel a certain distance. ―Szent-Györgyi⁽¹⁾
But his work in this area gives great insight into the function of methylglyoxal, since he had proposed that its anticancer effect was due to its ability to modify proteins to the extent of changing their conductivity.The energies available are insufficient to raise an electron from the ground state into the conduction band of proteins, the distance between the two being about 3 ev. This is probably the reason why my own suggestion, put forward two decades ago, that proteins act as semiconductors, bore no fruit, and so remained a dead duck. ―Szent-Györgyi⁽⁴⁾
He thought that the increase in protein conductivity with the addition of methylglyoxal was due to a Schiff base with lysine, although he hadn't actually proved this. More likely is through the reaction with arginine in which cyclic imidizoles are formed. These are also flourescent, and have now been well-characterized. They have also been recently shown to exert control on the cell's DNA→RNA transcription. This was done by Thornally.The previous chapter opens the possibility that it might have actually been MG or a closely related ketone-aldehyde that served as acceptor in the electronic desaturation of protein and so might have started up the development and differentiation in the a period. ―Szent-Györgyi⁽⁶⁾
Our studies demonstrate for the first time that methylglyoxal causes post-translational modification of a coregulator protein and that this modification affects gene expression. ―Thornally⁽¹⁴⁾
These imidizazoles were even isolated; they had been proven to exist. The amount of steps, confirmations, details, and proofs shown in Thornally's article is astonishing.⁽¹⁴⁾This, being the simpler case, may also be the more probable one. It involves the assumption that all the systems concerned have identical receptors for the common chemical signal. ―Szent-Györgyi⁽⁷⁾
It seems possible that a similar transfer of electrons plays a wider role in the various activities of the cells or the maintenance of their living state. It is still an open question how such a transfer of electrons takes place. ―Szent-Györgyi⁽³⁾
The electrons from photosynthesis must be made to do work. He envisions this as progressive charge transfer from one molecule to the next, with the emission of photons.Thus, the problem of energetics narrowed down to the question: How could a chemical potential be translated into a free electronic energy? [...] A tentative answer is suggested by my Fig. 7 in which the energy is released as electronic-free energy, which could emit a photon, or do work of any kind.―Szent-Györgyi⁽⁴⁾
Such things are known to occur in vitro. A charge transfer is not a reduction–oxidation reaction, since it involves the transfer of one electron only—often with the concomitant emission of light, or a photon.So, if an electron were transferred from a third substance to A, filling the empty place, then the electron on B could go on to a fourth molecule, or else, if one electron were taken away from the filled level of B (by transferring it, say, to 0₂), then the electron could drop from the excited level to the empty place on the ground level, emitting a photon, and giving a possible explanation for bioluminescence. ―Szent-Györgyi⁽⁴⁾
So he obviously has given some thought to the idea that immobilized resonant rings—such as in tryptophan and tyrosine—on a protein could be responsible for this process. He was well aware of the ability of both of these amino acids to undergo charge transfer reactions and phosphorescence. He had done experiments on this personally.We could go on playing with such ideas and imagine that we add to molecules A and B other substances, say, D, etc., A transferring an electron to B, B transferring from its ground state to the excited level of C, C to D, and so on. Now, if I would feed an electron into the empty place of A and take one out (by means of 02) from D, then all the electrons in the excited levels could shift one place, from B to C and C to D. This leads to a very amusing speculation: a biochemistry without chemistry, because the shift of one electron does not involve any rearrangement within the molecules which simply form a quantum mechanical scaffolding on which the electrons can cascade from molecule to molecule giving off gradually their energy. ―Szent-Györgyi⁽⁴⁾
And indoles, such as in serotonin and tryptophan, have a peculiar affinity to accept electrons from the flavin ring.We observed earlier that there occurred between indoles (serotonin) and FMN (flavinemononucleotides) a strong charge transfer-stronger than what might have been expected if one judged only by the P values of the reactants. ―Szent-Györgyi⁽³⁾
FMN is (as shown by the Pullmans) a fair acceptor, indole a fair donor. But "fair" is a poor compliment and does not explain such a strong reaction; there must be more to it. ―Szent-Györgyi⁽⁴⁾
I think he was right, there must be something more to this. Most biologists are handicapped by the fact they they are taught little about photochemistry.For this reason, therefore, a tryptophan solution of proper dilution, upon exposure to an unfiltered ultraviolet lamp, emits a distinct, though weak, violet fluorescence easily observable with the unaided eye. [...] This emission picture changed markedly for the aromatic acids when the solutions were made to 0.5 per cent in glucose prior to freezing. Tyrosine and tryptophan in 10⁻³ molar concentration gave short and prolonged afterglows, respectively. These emissions were of considerable intensity. ―Szent-Györgyi⁽⁹⁾
I am willing to bet that if he had known of the chemical structure of microtubles, and had seen the histological data as well as those obtained via X-ray crystallography, he would have recognized immediately that these were the very structure he had been looking for—the only suitable structure for long-range transmission.One of my difficulties with protein chemistry was that I could not imagine how such a protein molecule can "live." Even the most involved protein structural formula looks "stupid," if I may say so. If the atomic structure is only the backbone underlying the common energy levels, the thing becomes more likely. ―Szent-Györgyi⁽¹⁾
While most other branches of biochemistry make rapid progress, these gaps remain unbridged. It looks as if something must be missing from our knowledge and way of thinking without which no progress can be made on these lines. A whole dimension seems to be missing somewhere. ―Szent-Györgyi⁽³⁾
It's missing on purpose. He had very likely figured-this out later, after doing work with methylglyoxal and cancer.The situation is similar in most other biological processes and pathological conditions, such as the degenerative diseases. This suggests that some very basic information is missing. ―Szent-Györgyi⁽⁵⁾
And realizes that a diphosphonucleotide, such as NADH, should rightly be considered the fundamental unit of bioenegetics—and not changes of heat and entropy, his "delta effs:" ΔF = ΔH + ΔSWhat I was unable to understand was how the interaction of two members of the oxidative chain could create a high-energy P-O-P bond, and how this high-energy P-O-P bond could do work, how it could, for instance, make muscle contract, produce mechanical work in muscle, or electric work in nerve cells. My difficulty was this: a chemical reaction consists in the rearrangement of atoms within the system formed by two molecules, say, A + B ⟶ AB ⟶ C + D. Thermodynamics describes the change in useful energy by a ΔF. But how could a rearrangement, taking place within a closed system, do something useful outside? So, for me, these ΔFs were no more than bookkeeping items, and not currency with which to buy something. ―Szent-Györgyi⁽⁴⁾
So much was his puzzlemnt over how entropy and enthalpy inside of the phosphodiester could be made to do work on another molecule, he examined the electrical characteristics of ATP: namely, the ability of ATP the participate in charge transfer. This can determined by electron spin resonance.It seems probable to me that surprises may be in store on this line for the student of energy relations, and that the energy of DPNH also may be fed more directly than through ATP into the metastable living structure which has to be maintained. ―Szent-Györgyi⁽⁴⁾
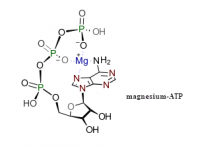
He had hopes that the extra phosphate in ATP—compared with ADP—would allow the terminal phosphate to curl-over and interact with the adenine ring, but there was little actually evidence of charge transfer.It is therefore of interest to note that, as has been shown by the Courtauld atomic model, folded configurations of ATP may exist in which the triphosphate group is in close proximity to the adenine. In one of these configurations it may be shown that the triphosphate group fits very neatly on the face of the adenine ring structure. In this folded configuration there may be appreciable overlap between the orbitals of the adenine and the triphosphate moieties. ―Szent-Györgyi⁽¹¹⁾
Perhaps he was missing magnesium? Adenosine triphosphate does have a peculiar affinity for Mg⁺, and they are commonly thought associated within the body. The complex has even been described as a "chelate."Several possibilities present themselves for understanding the weakness of the ATP signal. [...]
(5) The signal may be due to a transition metal impurity in the form of a metal-ATP complex. Judging from the procedure for the manufacture of ATP,4 such an impurity would be quite a large one. This possibility, though not likely, cannot be ruled out at present. ―Szent-Györgyi⁽¹¹⁾
 click to embiggen
click to embiggenA Russian embryologist, Alexander Gurwitsch, found that the parts of an organ or embryo could exert their stimulating or organizing influence on other cells even through a piece of glass, and by using different types of filter, he identified ultraweak ultraviolet rays as a medium of communication between cells. F.A. Popp and others are currently studying the integrating functions of ultraweak light signals. Guenter Albrecht-Buehler (who has an interesting website called Cell Intelligence) is investigating the role of pulsed infrared signals in cell communication. ―Peat⁽¹⁷⁾
Interesting side-note: Albert Szent-Gyorgyi had discovered actin but had been chased-out of Europe by Hitler.Electrical fields produced by cells, tissues, and organisms have been shown to influence cellular metabolism and physiology, and to influence growth patterns. Closely associated with cellular electrical fields are fields or gradients of pH and osmolarity, and all of these fields are known to affect the activity of enzymes, and so to create environments or fields of particular chemical concentrations.―Peat⁽¹⁷⁾
And he makes a Schrödinger-type connection (and also has an ö-umlaut in his name), by viewing animal metabolism as essentially a reversal of photosynthesis.This study led to the discovery of a new protein, which he discovered with I. Banga at the University of Szeged, Hungary. They called it "actin" because it made the inactive myosin act to contract. This discovery has an unusual history. It was never published because just when they were about to publish, Hitler occupied Hungary and Szent-Gyorgyi had to disappear "underground" separated from Banga and science. ―Szent-Györgyi⁽¹²⁾
We can thus drop these arrows and pull the two sides together, as in Fig. 4, completing the sketch with the main actor, the good old sun, which sends us its photons. Carbohydrate and fat are but a side line; if we leave them off, we have before us the essentials of the energy cycle of life, which consists of electrons being boosted up by photons, and then dropping back to their ground level through the living systems, giving up gradually their excess energy which then drives the living machinery. ―Szent-Györgyi⁽⁴⁾
Here is the analysis https://arxiv.org/ftp/quant-ph/papers/0208/0208053.pdfHow? Did they shine a light through a mouse's optic nerve or something?
Okay. It starts off with a well-worded paragraph warning about certain authors who engage in quantum woo and not science.Here is the analysis https://arxiv.org/ftp/quant-ph/papers/0208/0208053.pdf
Good thing this had been written before Massimo Cocchi's article, or else Georgiev might still be recovering from shock‼In the last two decades increasing number of researchers speculates that consciousness, which is often referred to as the last great mystery of science, should have quantum origins. However the amount of literature accumulating on the subject is either bad science or pseudoscience. The word “quantum” is used inappropriately by authors with little understanding of its actual meaning who are mostly interested in making their discourse sound more technical and scientific than it otherwise would be. Moreover, the very same writers have little or no knowledge of neuroscience and as a consequence their “quantum mind” proposals are already wrong at the moment of their conception. ―Georgiev
This is be no means eccentric. Technically, light is thought to flow concentrically through microtubulesOne of the statements [by Hameroff] is that microtubule-based cilia/centriole structures are quantum optical devices: “microtubule-based cilia in rods and cones directly detect visual photons and connect with retinal glial cell microtubule via gap junctions”. Though it is eccentric to suppose that microtubule based cilia can capture visual photons and microtubules can act as waveguides to transmit the photons to the cerebral cortex, ―Georgiev
I would assume that this is because they are they are between the microtubules or the rod cell and those of the optic nerve. I'm hesitant to continue since he thinks this is beyond comprehension....it is beyond comprehension why the retinal glia microtubules need to be involved in the process. ―Georgiev
I don't think Gilbert Ling and Ray Peat would agree with this series of events. This depends on "cyclic nucleotide-gated ion channels permeable for Na⁺ ions." This is essentially synonymous with Na⁺/K⁺-GTPase, a membrane pump powered through "high-energy" phosphate heat energy (ΔH). I am willing to bet that Szent-Györgyi would not have like this either, since it depends on multiple stages of diffusion: That of of Na⁺ ions, and that of the glutamate released as a consequence of this.Our eyes see the incoming light photons due to the existent photoreceptors located in the retina. The photoreceptors are responsible for the transduction4 of light into electrical signals, which are then delivered to the brain cortex, where we consciously perceive the visual images.
2.1.1 Photon transduction and signal amplification: The sensory transduction (the conversion of the incoming light into electric signal) takes place in the photoreceptors (rods and cones). It is a three stage process involving (1) photon induced isomerization of a pigment called retinal, subsequent to the absorption of a photon, (2) a biochemical cascade to amplify the incoming signal, and subsequent (3) alteration in the conductance of plasmalemmal cyclic nucleotide-gated (CNG) ion channels permeable for Na⁺ ions (see Figure 1). ―Georgiev
The term "conformation changes" is a massive cop-out, and commonly used when someone is too unsure, confused, or timid to provide a more detailed chemical mechanism. The explanation that best suits Vitamin A's function is "photobleaching:" Where the cis–trans isomerization of retinal merely obscures the light, almost like a diffraction grating, decreasing the sensitivity in bright light and increasing it in the dark. In my opinion, this is the primary function of vitamin A in the retina.If this molecule absorbs a photon, it undergoes photoisomerization forming straight chain version, known as all-trans-retinal. Alltrans-retinal unleashes a series of conformational changes in the protein opsin fragment producing metarhodopsin II, which is the activated form of rhodopsin. Most of the conformational changes occur in less than a millisecond, but the last transformation, from metarhodopsin II to metarhodopsin III, requires several minutes to accomplish. ―Georgiev
Cumbersome is an understatement. It's amazing that we can see at all.Metarhodopsin II initiates the second stage of phototransduction process via activation of an associated Gt molecule known as transducin. Transducin is a typical G-protein, composed of α, β and γ subunits, which is activated by exchange of guanosine diphosphate (GDP) for guanosine triphosphate (GTP) within its α-subunit. Upon activation the Gt α-subunit is transferred to and activates a phosphodiesterase (PDE) that hydrolyzes cytoplasmic cyclic guanosine monophosphate (cGMP) to guanosine monophosphate (GMP). Reduction in the concentration of cytoplasmic cGMP in the photoreceptor outer segment releases bound cGMP from the cyclic nucleotide-gated (CNG) ion channels. Dissociation of cGMP from the CNG ion channels initiates the final stage in the phototransduction process, the inactivation of the Na⁺ currents through these CNG channels in the photoreceptor outer segment. This complex multistage photochemical process might seem cumbersome, ―Georgiev
The brain is thought to run more or less on direct current. This carries less information than light. For confirmation of this, look at the byte transfer rate of fiber-optic cables vs electrical cables. Fiber optic signals can be modulated in many different ways. A channel can be modulated by frequency, by amplitude, by time, and by orbital angular momentum (not to be confused with the quantum mechanical term). These can be multiplexed onto the same waveform to carry far more information than a similar-sized DC wire at lower energy and higher speed.Thus the first essential feature of the retina is that it amplifies the photon signal and converts it into macroscopic electric currents. ―Georgiev
He has done no experiment. His refutation of Hameroff up to this point is simply based on a textbook Rube Goldberg Hypothesis of Photoconducion, a motley chimera created from the serial addition of ad hoc explanations.Up to this point we have explicitly formulated the two most important points (facts) about visual perception that should be kept in mind by any researcher: namely there is (1) amplification and (2) irreversible processing of the visual information in the retina. ―Georgiev
He admits that information is sent down the optic nerve, but he seems to think this is in the form of DC binary pulses and draws-upon prior articles with computer analogies. Still no mention of what structure within the axons themselves are responsible for carrying this current. Microtubules are the only continuous parallel structures within nerves, which are surrounded by non-conductive myelin. Szent-Györgyi (see above) and others proved that the conduction of direct current through a standard peptide backbone encounters a fairly high resistance, similar to rubber, with an experimentally-determined resistivity on the order of giga-ohm·meters. The photonic conduction of microtubules is yet to be determined, but experiments have confirmed that they do normally emit light. The lumen of microtubules are not opaque to the same extent that their backbones are resistive.Modern digital computers are based on digital electronic circuits within which the signals interact at gates that perform specific Boolean functions. All Boolean functions could be formed by various combinations of 3 elementary functions: AND, OR and NOT. These functions could be best appreciated by building a truth-table that illustrates all of the input-output relationships of a gate (cf. Mendelson, 1970; Kingsley, 1996). ―Georgiev
Tuszynski's articles would then seem on par with Massimo Cocchi's . . .The last proposal of “quantum” visual perception to be discussed is proposed by Tuszynski’s group (cf. Salari et al., 2008; Rahnama et al., 2009) and involves quantum teleportation of photons. The authors propose that the visual photons are quantum teleported to the brain cortex and they collapse in the cortex instead inside the retina. ―Georgiev
. . . and perhaps even Georgiev's himself.The input of visual information to the brain cortex is a multistage process, in which the initial stimulus is registered by photoreceptor cells, converted into electric currents that affect the membrane potential and subsequently into altered release of neuromediator (glutamate) through exocytosis. The bipolar, horizontal and amacrine cells process the obtained information using graded potentials, while the ganglion cells and the neurons from LGN process the converted into action potentials information using a kind of Boolean binary logic. ―Georgiev
And I don't see how vision could be pixelated. Is there any instance of a drug, vitamin deficiency, or brain injury causing one to see courser squares? All of the psychedelic indoles that I've had cause hallucinations based on smooth and colorful shapes. Consciousness is probably better modeled as complex waveforms, and vision can be seen as light flowing into the brain uninterrupted through microtubules inside the optic nerve.In 1970, it was being seriously proposed that memory was produced by the death of brain cells, in a manner analogous to the holes punched in cards to enter data into computers. The cultural dogma made it impossible to consider that learning could be associated with the birth of new cells in the adult brain. ―Ray Peat*
Just learned from Miles Mathis a few interesting things. As it turns out, Karl Popper delivered a crushing blow to the Copenhagen School by publishing a powerful 38-page logical critique.
It was pretty good, but his explanation for the double-slit experiment wasn't. He lifted an explanation from a textbook by Alfred Landé that doesn't really seem to explain it—the same explanation used by Mathis.
- Popper, Karl R. "Quantum mechanics without “the observer”." Quantum theory and reality. Springer, Berlin, Heidelberg, 1967. 7-44.
Could the effect simply be that only certain angles are refracted off of the inner slit, or repelled by the subtle electrical charge of the metal grid? This is a good article to look at.
The diffraction pattern doesn't "disappear" when you only use one slit. You have to see a panoramic image to notice this, but the effect is there. Even a single-slit produces diffraction patterns.
- Bach, Roger, et al. "Controlled double-slit electron diffraction." New Journal of Physics 15.3 (2013): 033018.
(But the article below is hard to explain.)
- Strekalov, D. V., et al. "Observation of two-photon “ghost” interference and diffraction." Physical review letters 74.18 (1995): 3600.
You're right. Mathis' double-slit article was the first one that I had read. I then went on a reading spree where I had read a few more Mathis articles and then found the Popper article. I didn't like Mathis' use of the messenger photons, wiped-it from my short-term memory, and later confounded his many other Popper affirmations and references contained in this article with his adherence to Popper's—or should I say Landé's—brief explanation of the double-slit effect.I just skimmed Popper's paper and I don't think his explanation is the same since he never used the charge field emitted by the material which contains the slit through which the particle passes or assigned the same particle mechanics, or even mass, to photons as Mathis. Like Mathis says, every atom of the material is emitting particles called' messenger photons' that make up the charge field and interact with the observed photon particle by changing its spin property, deflecting, and changing its energy; and this creates the diffraction.
a new virtual photon like Mathis,
Surface polaritons are waves which travel along the surface of metals which can be best illustrated by an electric field intensity distribution
Those waves actually are the photons, after they have been captured by the metal surface. They then can be emitted later at a lower frequency. The angle of incidence determines whether or not a photon is captured and guided along a metal surface. If you wan't to know how they behave around two slits, you could perhaps read these articles:And how exactly are these waves interacting with the photons to create the diffraction pattern?
University deans probably like it since it makes physics sound fun and mystical . . .They're not just science hacks anymore, they are brilliant philosopher–artists who have serendipitously discovered the most profound secrets of life simply from shining light through two slits. Join Calctech Physics Department: Be the shaman of science.I was like: Whooa! Mind-blown, Seth. ―Cecily Strong
I'll have to take a look at Mathis' article on photons. I found the one on π to be especially good. It was shocking and I'm fairly certain he's 100% correct. I think you could could say that he had effectively proved it.Feynman was fond of saying that all of quantum mechanics can be gleaned from carefully thinking through the implications of this single experiment. ―Wikipedia
linseed oil really is bad-tasting.
Someone needs to tell the chickens this
Those waves actually are the photons, after they have been captured by the metal surface. They then can be emitted later at a lower frequency. The angle of incidence determines whether or not a photon is captured and guided along a metal surface. If you wan't to know how they behave around two slits, you could perhaps read these articles:
The idea was that there are at least four ways that light interacts with metal: By reflection, Compton Effect, Photoelectric Effect, and by becoming surface plasmon polaritons. So nobody should be troubled by the fact that shooting electrons one-by-one also creates regular diffraction patterns. The simple answer might be that the light is reflected, repelled, absorbed–emitted, and absorbed–travelled–emitted by the slit's edge and inner surface.
- Zia, Rashid, and Mark L. Brongersma. "Surface plasmon polariton analogue to Young's double-slit experiment." Nature nanotechnology 2.7 (2007): 426-429.
- Schouten, H. F., et al. "Plasmon-assisted two-slit transmission: Young’s experiment revisited." Physical Review Letters 94.5 (2005): 053901.
I think it only seems bizarre when you originally imagine light waves as ocean or sound waves. Below is Richard Feynman making water analogies, referring to the mask as "barges" lined-up with gaps and the electron detector as "buoys." This is setting-up the audience for later "gotcha" moments,
. . . in which the viewer is is left astonished. This only happens because they were given given exaggerated drawings and forced to accept water-wave analogies. Like a magician Richard Feynman delivers mind-blowing conclusions one after another which logically follow from the ocean wave analogy and Heisenberg's so-called "Laws," and delivers the prestige a bit after the 50 minute mark.
University deans probably like it since it makes physics sound fun and mystical . . .They're not just science hacks anymore, they are brilliant philosopher–artists who have serendipitously discovered the most profound secrets of life simply from shining light through two slits. Join Calctech Physics Department: Be the shaman of science.
I'll have to take a look at Mathis' article on photons. I found the one on π to be especially good. It was shocking and I'm fairly certain he's 100% correct. I think you could could say that he had effectively proved it.
But it's discoverer speaks as if were real:Senescence-associated beta-galactosidase (SA-β-gal or SABG) is a hypothetical hydrolase enzyme that catalyzes the hydrolysis of β-galactosides into monosaccharides only in senescent cells. Senescence-associated beta-galactosidase, along with p16, is regarded to be a biomarker of cellular senescence. —Wiki
But the discoverer also refers to the enzyme as a verb, as well as a hypothetical noun.We show that several human cells express a β-galactosidase, histochemically detectable at pH 6, upon senescence in culture. [...] Senescent human fibroblasts expressed a β-Gal that was detected in single cells by X-Gal, which forms a local blue precipitate upon cleavage (24), independent of DNA synthesis measurements. [...] Cells from all three embryonic layers expressed β-Gal upon senescence in culture.† —Dimri
Enzymes are often named before they are even isolated, based on their activity alone. They are sometimes known as enzyme–verbs before they are enzyme–nouns. But even speaking about every chemical reaction as enzymatic seems premature in many cases, as many chemical reactions happen nonenzymatically.Early passage adult melanocytes grew well in culture, yet >90% expressed pH 6 β-Gal activity. —Dimri
But it seems to be the fashion among biologists to ascribe every single chemical reaction to an enzyme, even one which can happen spontaneously. Every phosphorylation, acetylation, hydroxylation, dehydrogenation, and . . . even reductions. This leads to extremely complex reaction cascades, in which areas of phosphorylation are so impossibly crowded by diverse apparitions of kinases and phosphodiesterases that it makes you wonder how space can be made available for everyone's hypothesized mechanism. But everyone must have their glory, right? Everyone wants to name a molecule or pathway, to stake their name on it like like Buzz Aldrin impaling a flagpole on the Moon.A plausible primordial base can be traced for glycolysis and the PPP, as several of their reactions can be replicated with metal catalysts, in particular Fe(II), under conditions reproducing the ocean chemistry of the Archean world [8]. Fe(II) was broadly available before oxygenation of the early Earth [9], implying a scenario for the first glycolytic enzymes being simple iron-binding RNA or oligopeptide molecules, which would have possessed the potential of enhancing many reactions now found in central metabolism [7,8] (Box 1). —Keller
Here, we report iron-catalyzed deallylations that selectively cleave functionalized ethers under mild conditions . . . ―Gärtner
Yeah. I always correct people on that. You could have noticed my penchant for not using genericizing trade names, even to the point of saying such things and "transparent tape" if it happens to be from 3M® and not Scotch®. Here is a list of quite a few generic trade names which have invaded the lexicon like a clown who had snook under the tent flap, or . . . like linoleic acid being force-injected into reconstituted cork tile.@schultz ― Another product made with linseed oil is true linoleum (not vinyl) where it is mixed with cork. Yes it is good for making floors lol.
I went read a few lipofuscin articles a few months ago. The best ones, in my opinion, are from Terman and Brunk. Here is a full-text link to one of the review articles.Traditionally the oil is boiled which helps kickstart he polymerization process. Other ways they "boil" linseed oil are by blowing oxygen through it or by adding heavy metals to the oil. Now ponder that for a bit while thinking about what lipofuscin is. Similar processes are happening inside the body. Lipofuscin is synonymous with aging.
From what I remember Brunk and Terman saying: Lipofuscin accumulates mainly in non-renewing cells. The first time that I had become aware of such non-mitotic cells was from them. Some cells always divide and dilute the lipofuscin, but some cells do not. Some cells of the central nervous system accumulate the most lipofuscin since they don't have the benefit of dividing at a high rate.The question is, does lipofuscin accumulate in cells that have already stopped replicating or does lipofuscin itself cause the cell to not be able to replicate and/or repair itself? I suspect the latter.
 click to embiggen
click to embiggen click to embiggen
click to embiggenWhat about the iron oxide in sunscreens to change the white of the zinc oxide to the tone of the skin?Artists have noticed this. Some oil paint colors dry faster than others; some metal pigments increasing the "drying" rate of the oil. Windsor & Newton have kindly tabulated the drying time of their oils. You will notice that the faster drying colors are the ones with transition metal pigments, and the slower ones are the metal-free polycyclic fluorophores such as alizarin crimson. Iron tops the list, but iron is not explicitly named (hint: iron oxide is found in burnt sienna).
I don't like the idea, and this is the first time that I heard about such a thing.What about the iron oxide in sunscreens to change the white of the zinc oxide to the tone of the skin?
It must affect the reflective properties of zinc oxide and at the same time interact with radiation and skin unsaturated oils.I don't like the idea, and this is the first time that I heard about such a thing.